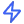Real Artificial Intelligence for Clinical Trials
Datacapt AI embeds practical artificial intelligence into clinical trials to accelerate study design, data review, monitoring, audit readiness, query management, and multilingual execution, without compromising compliance.
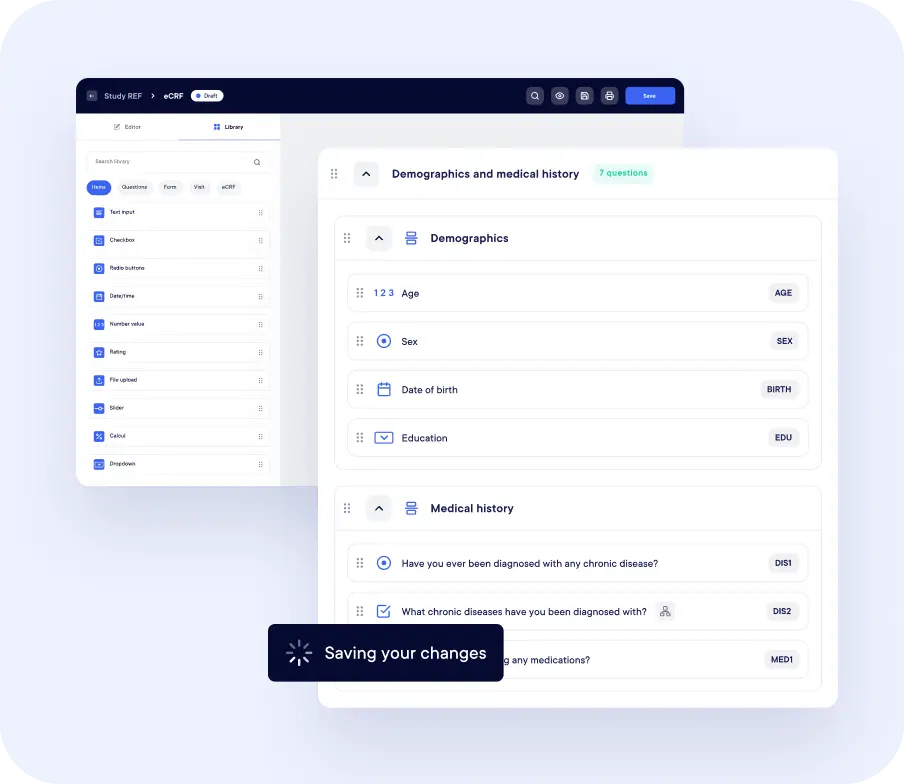
AI Powered Study Design

AI Powered Patient and Site Review
AI Driven Monitoring
.webp)
From AI Assisted to AI Enabled Clinical Trials
It is a foundation for continuously improving how clinical trials are designed, executed, and monitored.
Seamlessly translate eCRFs, ePROs and study content. Apply updates in real time while preserving data integrity across all environments.
Monitor every change from build to export with AI that flags inconsistencies and ensures a pristine audit trail.
Transform live study data into clear, actionable reports. Datacapt AI continuously aggregates and updates study, site, and patient-level reports.
Break language barriers in global trials. Support sites in +125 languages, enabling instant communication and faster data resolution.
Efficiency meets expertise. All AI recommendations remain reviewable, editable, and fully validated by your teams.
Datacapt AI operates within validated and compliant infrastructures.Patient PII is protected, never exposed, and always handled in line with regulatory expectations.
Frequently asked questions
Datacapt AI is a set of artificial intelligence capabilities natively embedded in the Datacapt eClinical platform. It supports key clinical trial activities such as study design, data review, monitoring, query management, audit trail review, and translation. Datacapt AI assists users by identifying patterns, suggesting actions, and improving efficiency while keeping full human control and regulatory traceability.
Yes. Datacapt AI operates within the validated Datacapt environment and follows strict principles of traceability, transparency, and user control. All actions are logged, auditable, and aligned with clinical research compliance frameworks. AI outputs are explainable and can be reviewed at any time.
No. Datacapt AI is designed to support clinical teams, not replace them. All AI generated suggestions, queries, and insights require human review and validation. Decision making, approvals, and accountability always remain with qualified clinical professionals, in line with regulatory expectations.
Yes. Datacapt AI supports international studies by enabling instant translation of eCRFs, ePROs, queries, and study content. This helps ensure consistent terminology, faster communication with sites, and smoother execution across multiple countries and languages, while still allowing human review when required.
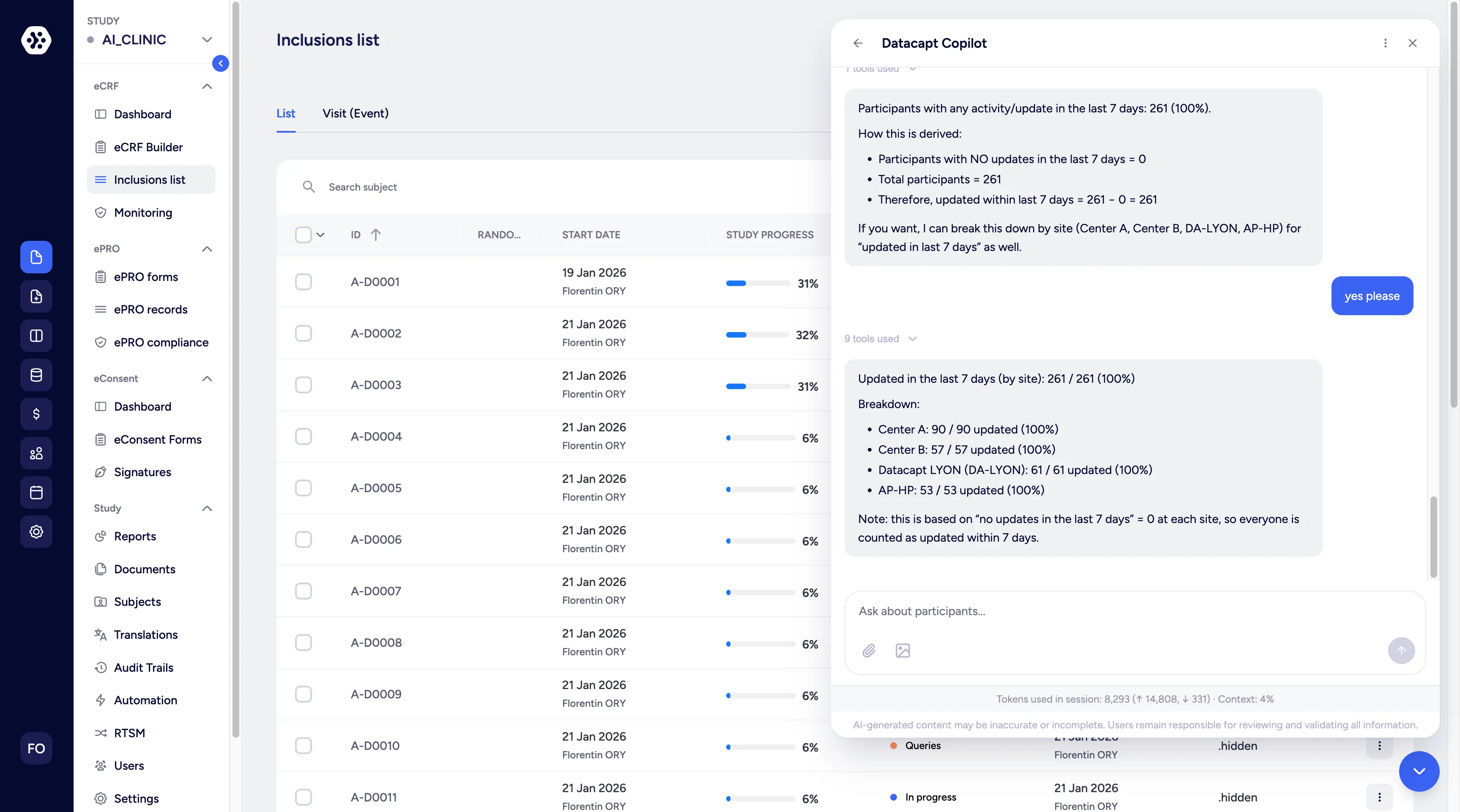
Datacapt AI assists with eCRF design by suggesting form structures, fields, edit checks, and validations based on protocol content and therapeutic context. This helps reduce study build time, improve consistency.
No. Datacapt AI does not generate or alter clinical data. It analyzes existing data and workflows to provide suggestions, alerts, and insights. All data entry, changes, and approvals remain under full human control and are captured in the audit trail, in accordance with clinical regulations.
Datacapt AI is purpose-built for clinical trials and embedded directly into the Datacapt eClinical platform. Unlike generic AI tools, it is designed around clinical workflows, regulatory constraints, and data integrity requirements, ensuring that AI delivers operational value without compromising compliance or control.
📞 Ready to Experience Datacapt AI?

Built for Trials.
Powered by Trust.
Experience the Difference.
Blog & News Datacapt
News, Articles, Resources et Tutorials.