Smart, Adaptive Randomization for Clinical Trials
Datacapt Randomization is a flexible, powerful module that simplifies even the most complex allocation strategies, fully embedded within the Datacapt EDC. With an intuitive interface and rapid setup, it gives your team the control, compliance, and speed they need, without the complexity.
.webp)
Join a Powerful Community Driving Change thanks to Datacapt





Datacapt turns randomization into a seamless part of your study workflow.
Randomization Made Simple
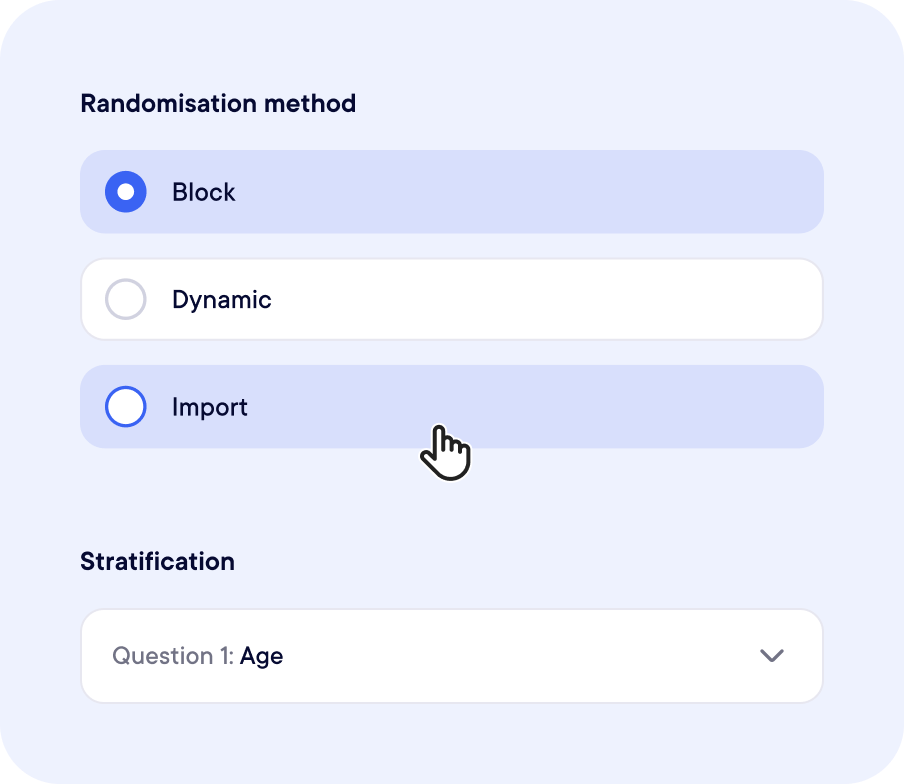
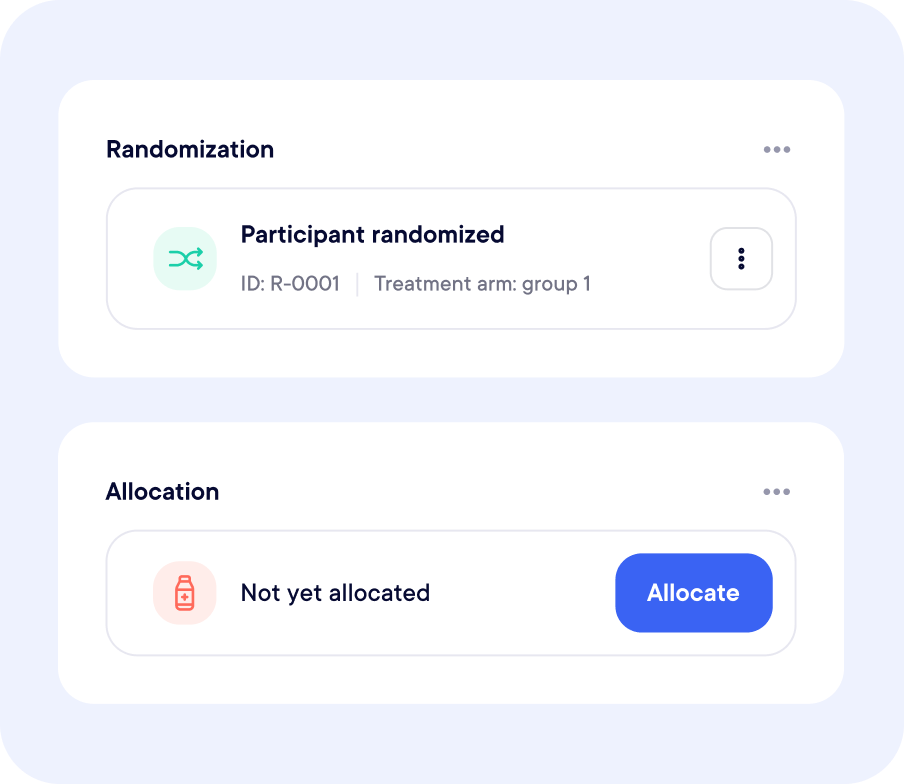
Fully Embedded in Your Study Workflow

Ready to Simplify Randomization?
Request a demo and see how Datacapt brings powerful, protocol-driven allocation strategies directly into your study environment—without complexity.
Frequently Asked Questions
We support block randomization, stratified randomization, dynamic randomization (minimization), and more.
Yes. All allocation logic is fully validated under GCP and 21 CFR Part 11 guidelines. We support advanced methods like Pocock-Simon minimization to ensure balanced treatment arms, with full documentation, audit trails, and compliance-ready processes.
Yes. Our no-code randomization module allows changes (e.g., ratio, strata) with versioning and audit control—ideal for adaptive designs.
Yes, Randomization and supply modules are linked in real-time to prevent errors and streamline logistics.
Other question
What do customers say about Datacapt ?
Find out why 200+ companies place their trust in the Datacapt
Platform to manage their clinical trials.







Built for Trials.
Powered by Trust.
Experience the Difference.
Blog & News Datacapt
News, Articles, Resources et Tutorials.









