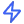Electronic Data Capture
for Modern Clinical Studies
Datacapt EDC eCRF makes it easy to build studies, collect, validate, and manage clinical trial data from any source.
Designed for all types of protocols, build studies in days, ensure cleaner data, and save significant time and effort.
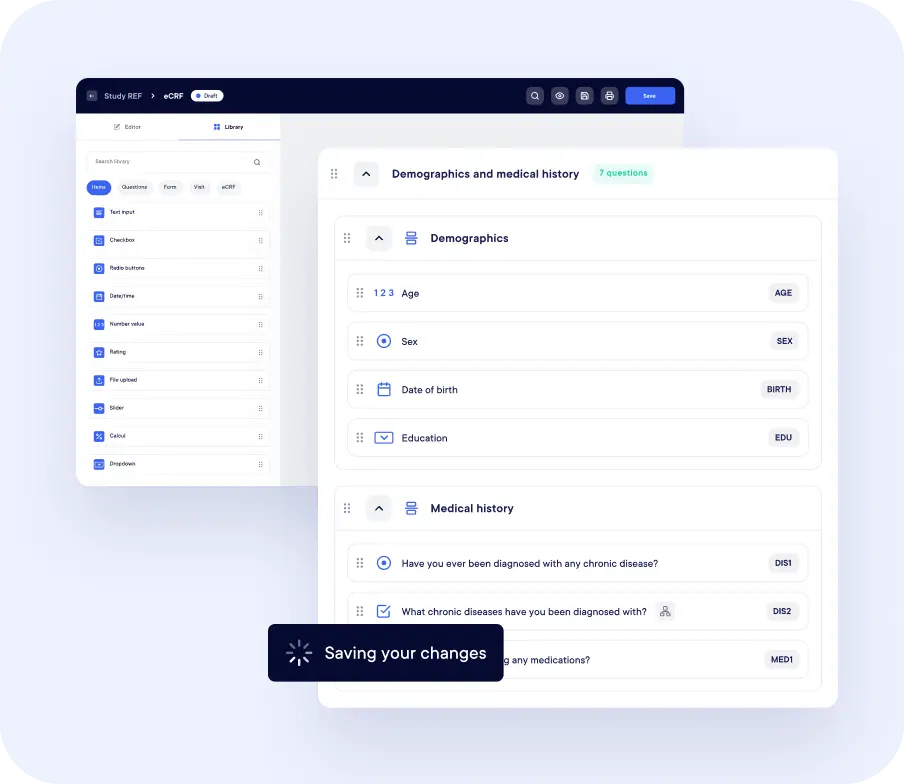
Our user-friendly platform is trusted worldwide for clinical study data management





Tired of Clinical Data Management Headaches?
Discover What a Modern EDC/eCRF Can Do.
Datacapt EDC eCRF is a cloud-native, intuitive, and AI-assisted solution designed from day one to empower data-driven research.
From first visit to last patient out, your teams move faster, with fewer clicks, cleaner data and no tech headaches.
Build without limits
Stay Ahead with Study Insights
Get cleaner data with less effort
.webp)

Experience how Datacapt redefines clinical data collection.
Datacapt isn’t a legacy system, it’s the next generation.
Everything you need and more
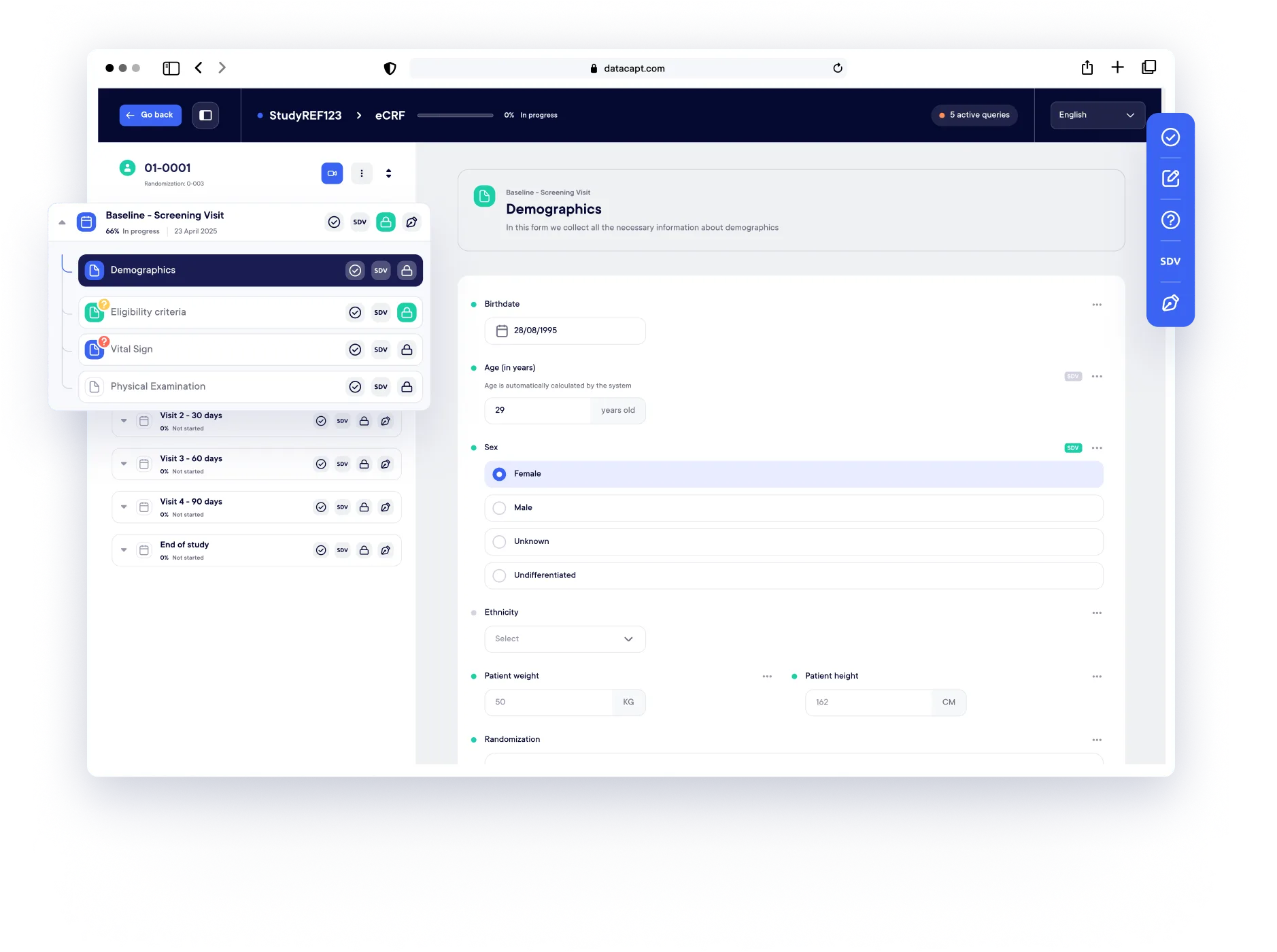
Implement protocol amendments, form updates, or logic revisions. No redeployment. No data loss.
From form building to data export, Datacapt eCRF empowers your team to configure, run, and update studies
Secure, precise, and compliant. Define who can view, edit, or lock data with robust role management.
Run global studies with +85 languages so your sites and patients can interact in their local language.
Get up and running in days, not weeks. Datacapt’s eCRF is designed for ease, from first login to first patient in.
Your data. Your rules. Our eCRF is natively built on the CDISC ODM format, ensuring full interoperability and regulatory compliance from day one.
Frequently asked questions
Datacapt EDC is an electronic data capture application that allows CROs, Sponsors and Academic Research to design and build data collection forms and enables patient data collection from sites efficiently and securely.
Designing eCRFs is intuitive and 100% no-code with a visual builder. No technical skills are needed, and you can use pre-built templates or customize forms effortlessly to suit your study.
Yes, Datacapt is fully compliant with 21 CFR Part 11 and other data privacy regulations. It’s fully validated, auditable, and audit-ready at all times. Validation documentation is available on request.
Yes. Every data entry, change, and user action is audit-trailed in real time. Electronic signatures are Part 11-compliant, timestamped, and fully traceable—meeting all regulatory expectations.
Your data is protected using end-to-end encryption—both at rest and in transit. We use AES-256 encryption, secure HTTPS protocols, and enforce strict data access policies across the platform. Data is hosted on ISO 27001-certified cloud infrastructure with geographic redundancy.
You can assign roles and permissions at a granular level, from data entry and monitoring to database lock and export. Each user only sees the data and features they need, ensuring security and clarity.
We support exports in CSV, Excel, PDF, and SDTM formats. You can export data on-demand at any point. Exports are secure, audit-logged.
Most studies can be built and deployed in less than one weeks using our form builder and template libraries.
📞 Ready to Experience It?
What do customers say about Datacapt ?
Find out why 200+ companies place their trust in the Datacapt
Platform to manage their clinical trials.







Built for Trials.
Powered by Trust.
Experience the Difference.
Blog & News Datacapt
News, Articles, Resources et Tutorials.