Smarter Clinical Supply. Delivered with Precision.
Datacapt Trial Supply Management brings real-time visibility and control to clinical supply logistics. Fully integrated with randomization and EDC workflows, it automates the entire supply chain, from kit assignment to site inventory, reducing risk, cost, and delays.
Join a Powerful Community Driving Change thanks to Datacapt





Say goodbye to spreadsheets and disconnected systems.
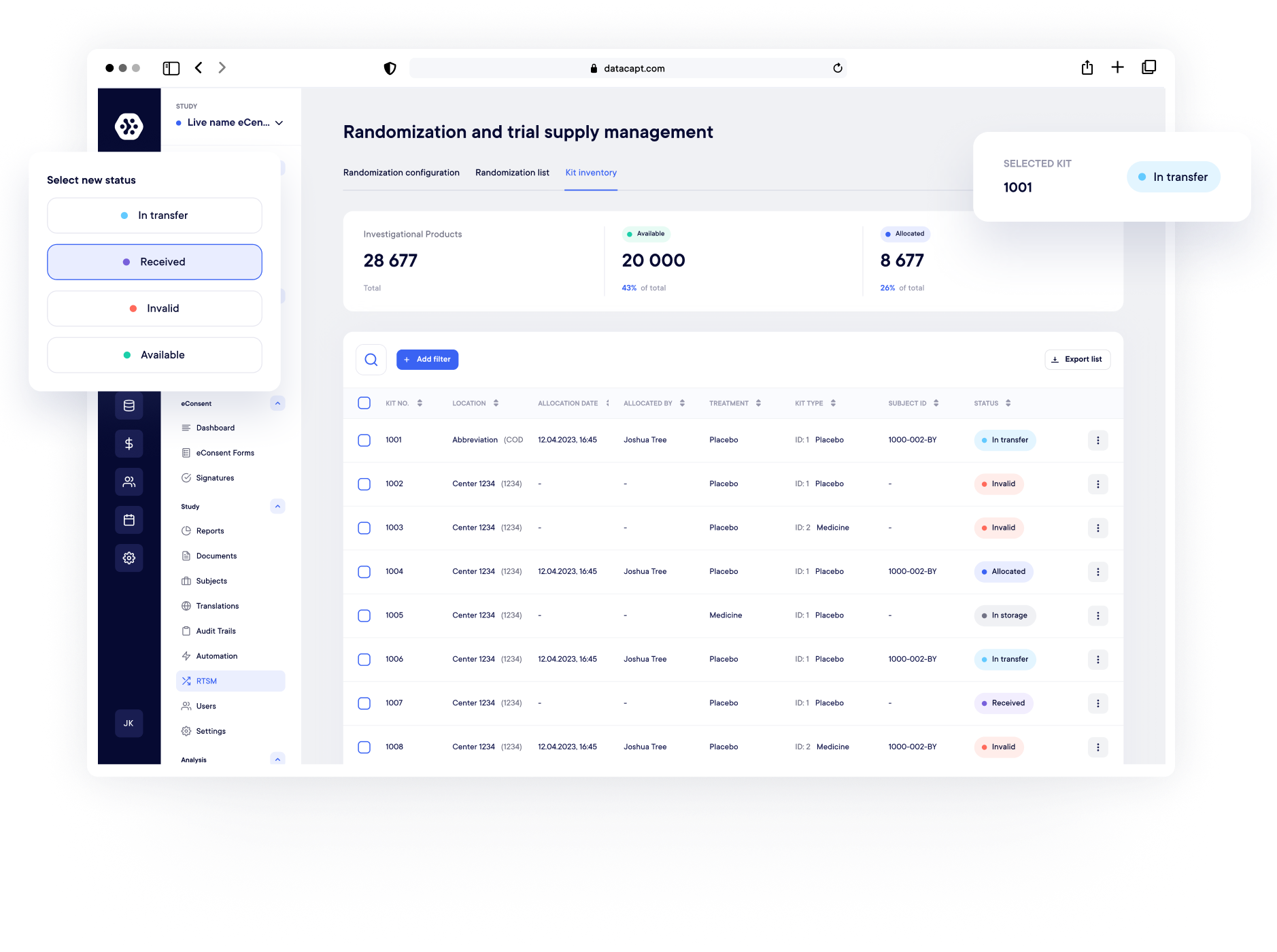
End-to-End Supply Management & Logistics

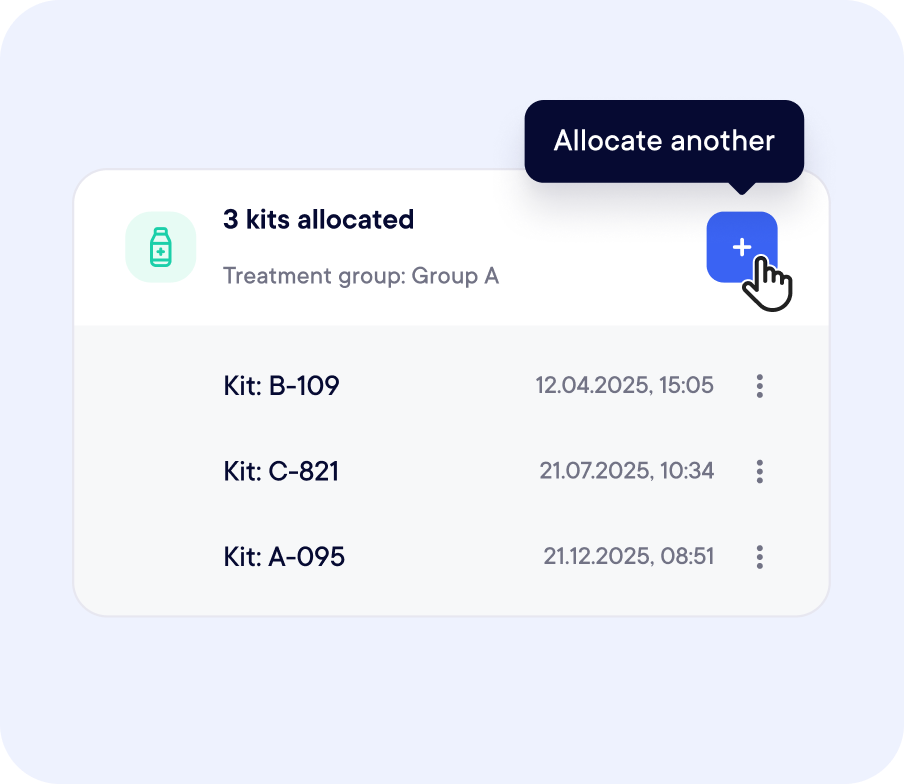
Integrated with Randomization and eCRF

Ready to Streamline Your Clinical Supply Chain?
Book a demo to see how Datacapt simplifies supply management with real-time control, seamless integration, and built-in compliance, designed for the demands of modern trials.
Frequently Asked Questions
Trial supply management (TSM) refers to the planning, tracking, and delivery of investigational products to clinical sites and patients. It ensures that the right supplies reach the right place at the right time.
Yes. Datacapt offers an integrated RTSM module that combines real-time subject randomization with dynamic supply tracking and automated kit assignment—removing the need for third-party tools.
Yes. We support fully blinded, open-label, and mixed studies with masking controls and secure role separation.
Absolutely. It’s GxP-compliant and meets 21 CFR Part 11 standards, with full audit trails, validation, and access control.
Absolutely. Datacapt provides real-time visibility of kit status at site and depot level, including stock levels, expiry dates, shipments, and returns.
Investigators can perform one-click emergency unblinding based on pre-approved permissions. Every unblinding action is logged with timestamp, user role, and justification to ensure audit-readiness.
Other question
What do customers say about Datacapt ?
Find out why 200+ companies place their trust in the Datacapt
Platform to manage their clinical trials.







Built for Trials.
Powered by Trust.
Experience the Difference.
Blog & News Datacapt
News, Articles, Resources et Tutorials.









