Book a demo
Book a live 1:1 demo with one of our expert.
Trusted by 200+ Clients Worldwide
7 500+ Clinical Trials

Simplified Clinical Trial Management

100% Compliant and Secure Solution

A Complete and Agile Platform






Why did Median Technologies adopt Datacapt eCRF for their studies?
“Median Technologies' team found that utilizing Datacapt brought several advantages, including real-time data entry monitoring, streamlined eCRF management, and comprehensive query tracking and resolution.” - Louis Chapotot, Senior Clinical Project Manager

Median Technologies is an innovative Health Technology company. They deploy proprietary artificial intelligence, computer vision, and signal processing technologies to develop imaging tests and services, addressing life-threatening unmet medical needs. They transform medical images into meaningful, actionable insights to help better diagnose, treat, and monitor patients.
Median Technologies is headquartered in France (Sophia-Antipolis, South of France) with subsidiaries in the United States (Boston area) and China (Shanghai).
Context
Teams at Median Technologies were looking for a reliable solution to structure data collection for retrospective studies. These studies aimed to validate internally developed medical image analysis algorithms. They needed a platform that was secure, compliant with regulatory standards, and flexible enough to centralize heterogeneous data from multiple sites. The challenge was to organize information efficiently while ensuring full traceability and offering a user-friendly experience.
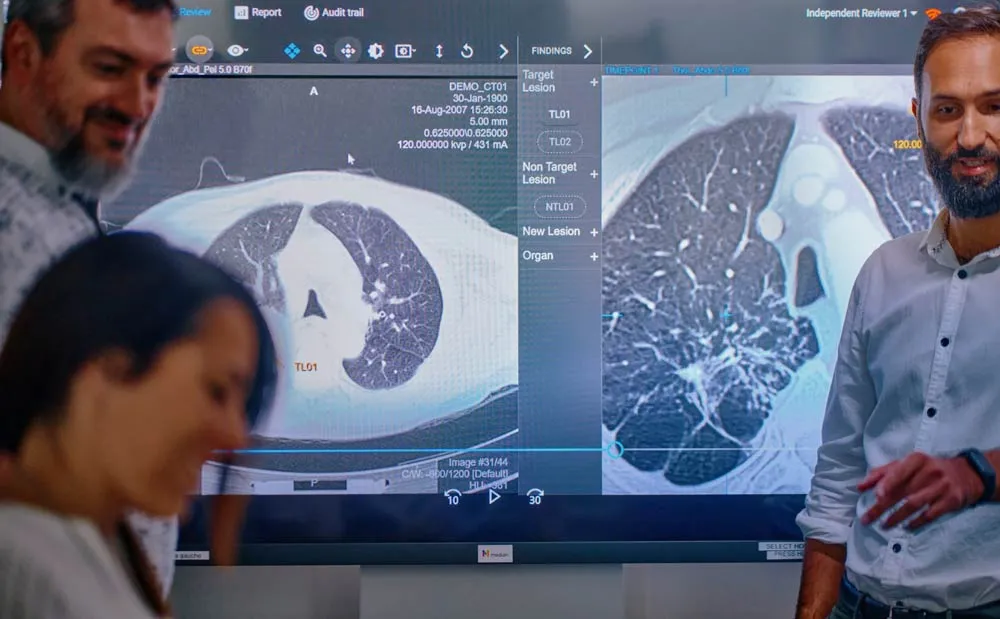
The challenge
Median Technologies encountered several challenges, including:
- Complex Radiological Reports: Analyzing radiology reports from different sites, and extracting information of interest from the reports is challenging.
- Structuring Reports: Providing a structured format for the reports was essential, as the data comes from different countries and different sites.
A change to their habits
Before implementing Datacapt, Median Technologies relied on their partners’ eCRFs.
However, it limited their ability to follow the data in real time during the data entry process. Besides, it also limited the ability to update the eCRF when minor changes were needed.
.webp)
The solution
3 aspects that convinced Median Technologies to choose Datacapt
1. Data Structuring
They needed a tool to help structure their clinical data and radiological reports, ensuring that the information of interest was collected. It significantly improved their data collection and management process.
2. Audit Trail
For clinical validation studies, an audit trail is mandatory. Datacapt provided them with direct access to a complete and readable audit trail, fulfilling this requirement.
3. Meeting Criteria
Datacapt met all the criteria set by Median Technologies, including security requirements, fast and easy implementation, a validated and robust platform, an intuitive and user-friendly interface, real-time reports and progress monitoring, efficient user training, and responsive customer support.
An effortless implementation
The implementation and internal validation of Datacapt proceeded smoothly.
Median Technologies provided training to all users of the eCRF platform and issued training certificates. They also updated their processes to integrate the use of Datacapt.
Tangible benefits
A change seen positively
Median Technologies’ team found that utilizing Datacapt brought several advantages, including real-time data entry monitoring, streamlined eCRF management, and comprehensive query tracking and review.
Taking control of the data collection process enabled them to manage and analyze the data more effectively. Datacapt allowed them to create eCRFs tailored to their specific data collection objectives.
They saved time and much more
Datacapt enabled Median Technologies to focus on different tasks during their studies.
Although some time was dedicated to user training, the platform’s intuitive and user-friendly nature facilitated smooth training. Data entry monitoring became more manageable, and overall data handling became simpler.
Leveled up their security
Median Technologies considered a high level of security crucial.
They required an eCRF provider that guaranteed strong security measures and traceability. An audit trail was a mandatory requirement for their studies. Datacapt fulfilled all their security and compliance needs, being a Validated System, certified ISO 27001 and 21 CFR Part 11 compliant, as well as GDPR compliant.
Before
- The complex nature of analyzing all reports and extracting information.
- Tedious report structures and data exchange between different sites.
- Difficulties in tracking the data entry process in real time.
- Limited ability to make necessary changes to the eCRF when needed.
After
- A centralized and secure platform to structure the forms and ensure data integrity.
- Enhanced security through a validated solution that meets all regulatory requirements.
- Improved visibility with real-time reporting and progress monitoring.
- Simplified data handling thanks to a user-friendly and easy-to-use - solution.

“Based on our positive experience, Median Technologies would definitely recommend Datacapt to other research centers for their clinical trials. The platform's ease of use, real-time monitoring capabilities, and overall benefits make it a valuable tool for research endeavors.”

Conclusion
By choosing Datacapt, Median Technologies found a solution perfectly suited to the needs of their retrospective studies and algorithm validation workflows. The platform enabled them to structure data efficiently, gain better visibility and responsiveness, while ensuring full compliance with regulatory standards. Thanks to its intuitive interface, fast setup, and responsive support, Datacapt has become a concrete driver of operational efficiency for Median’s clinical teams.


