For several years, teleconsultations in medicine have been developed in order to facilitate patients’ access to care. Thanks to the health situation and based on telemedicine, cosmetic clinical studies in teleconsultation are increasing.
They offer organizational optimization in management:
Deadlines
Number of subjects included
Multicentric studies location
These clinical studies in teleconsultation are considered when the CROs have the necessary IT tools to allow :

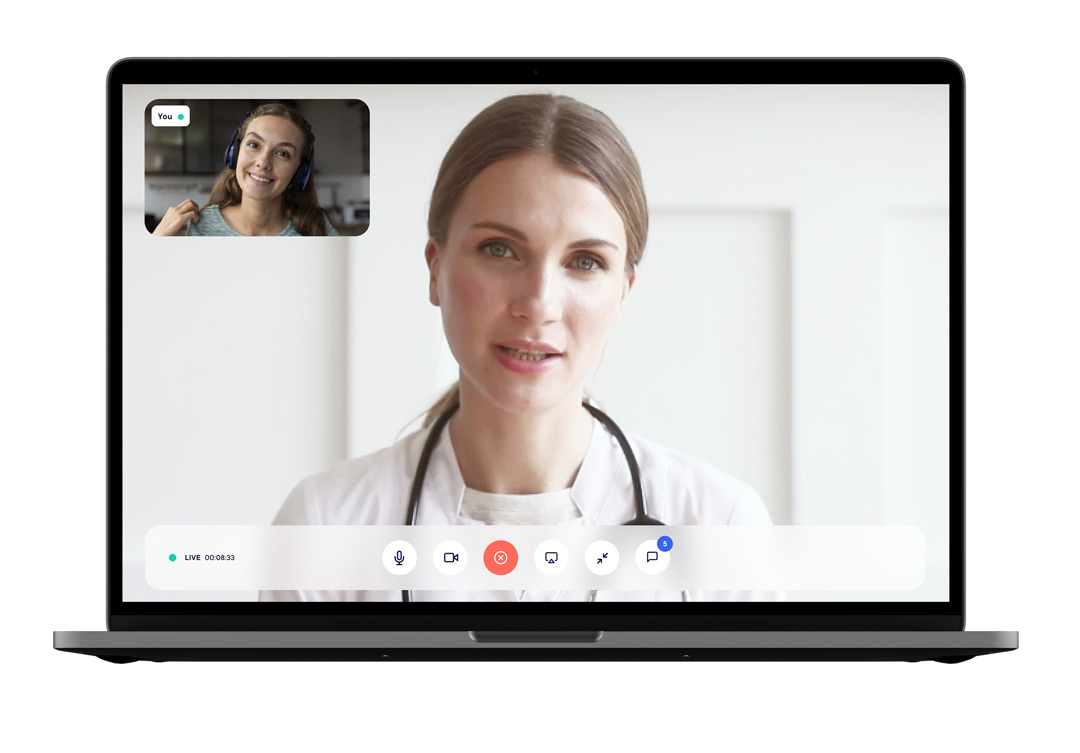
eConsult: A solution to carry out your clinical trials in teleconsultation with “tele-evaluation“
This new approach, which is currently being developed, meets many demands from CROs as it :
Datacapt’s eConsult allows you to:
- Nominative control of subjects (unique anonymous identifier)
- Integrated and secure high definition video-consultation
- Explanation of the context and the course of the study
- Electronic signature of consent in video
- Personalized follow-up of each subject
- Remote evaluation for your clinical studies (data collection)
Thus, although the study is conducted remotely, the volunteer is truly supported, which improves his or her commitment and compliance with the rules of the protocol.
HD and secure tele-consultation directly integrated into the solution
The investigator and technician can have direct access to the eConsent, eCRF, and ePRO for each subject. Study progress and tasks to be performed are easily tracked.
Documents exchanged between the subject and the investigator are secured and encrypted in order to comply with the regulations and to guarantee total confidentiality of the data.
Sponsor accesses can be opened to exchange study data in complete security and confidentiality.
A complete and tailored solution
In order to allow you to carry out your clinical tests in teleconsultation or on-site in the best possible conditions, Datacapt offers you modules that meet your needs in every way:
In terms of security and compliance, our modules are validated through these different steps: procedures, risk analysis, tests, validation report… and compliance with the regulations and standards applicable to your clinical studies: GDPR, ICH GCP E6 (R2), 21 CFR PART 11, HDS (Health Data Storage)…




