Seamlessly master your clinical trials with our scalable EDC & CTMS platform. Build, collect, monitor, share, and manage data management – all in one user-friendly, secure, and compliant platform. Boost productivity and data quality with our unified eCRF, ePRO, eConsent, Recruitment, and scheduling solutions.

One Cloud Platform, Endless Possibilities.
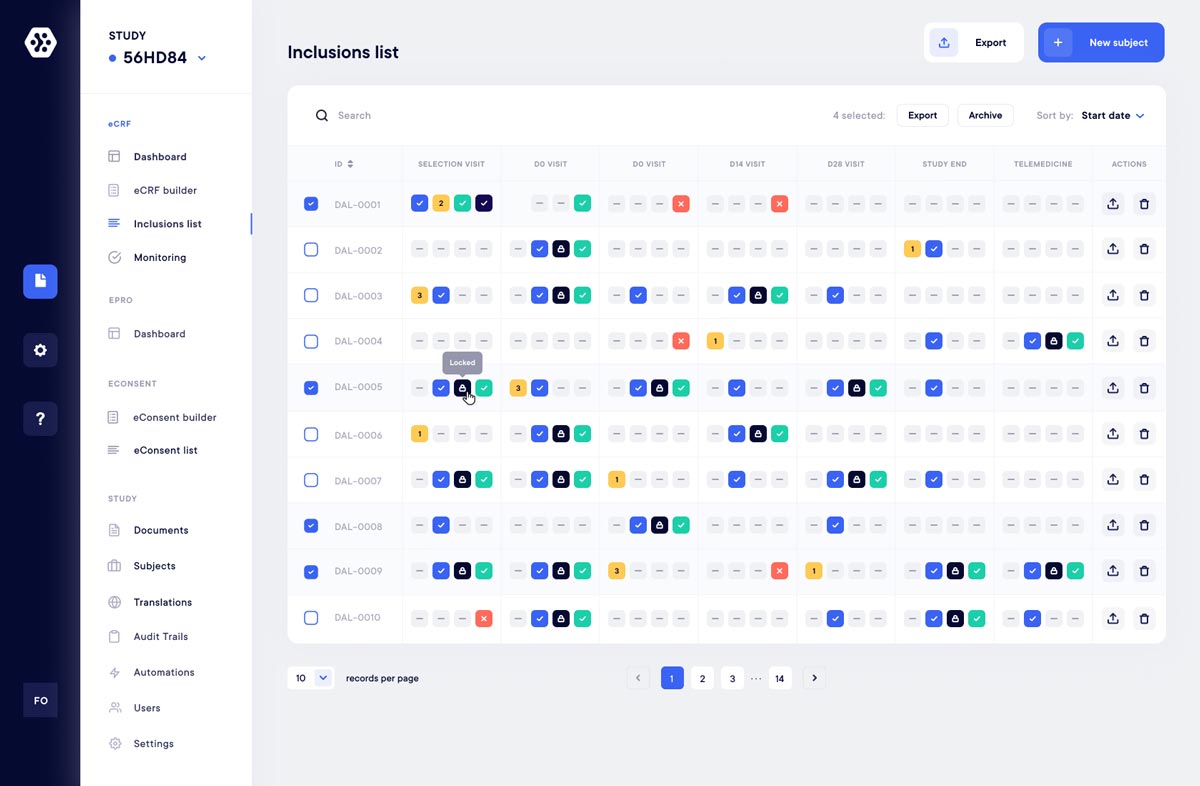
eCRF/eSource - EDC Platform
eCRF/eSource, a complete toolbox for clinical data collection
Quickly create your study forms with an infinite number of possibilities. Easily collect and monitor your studies and data in real time (Progress, Queries, Status, Automations, etc.).
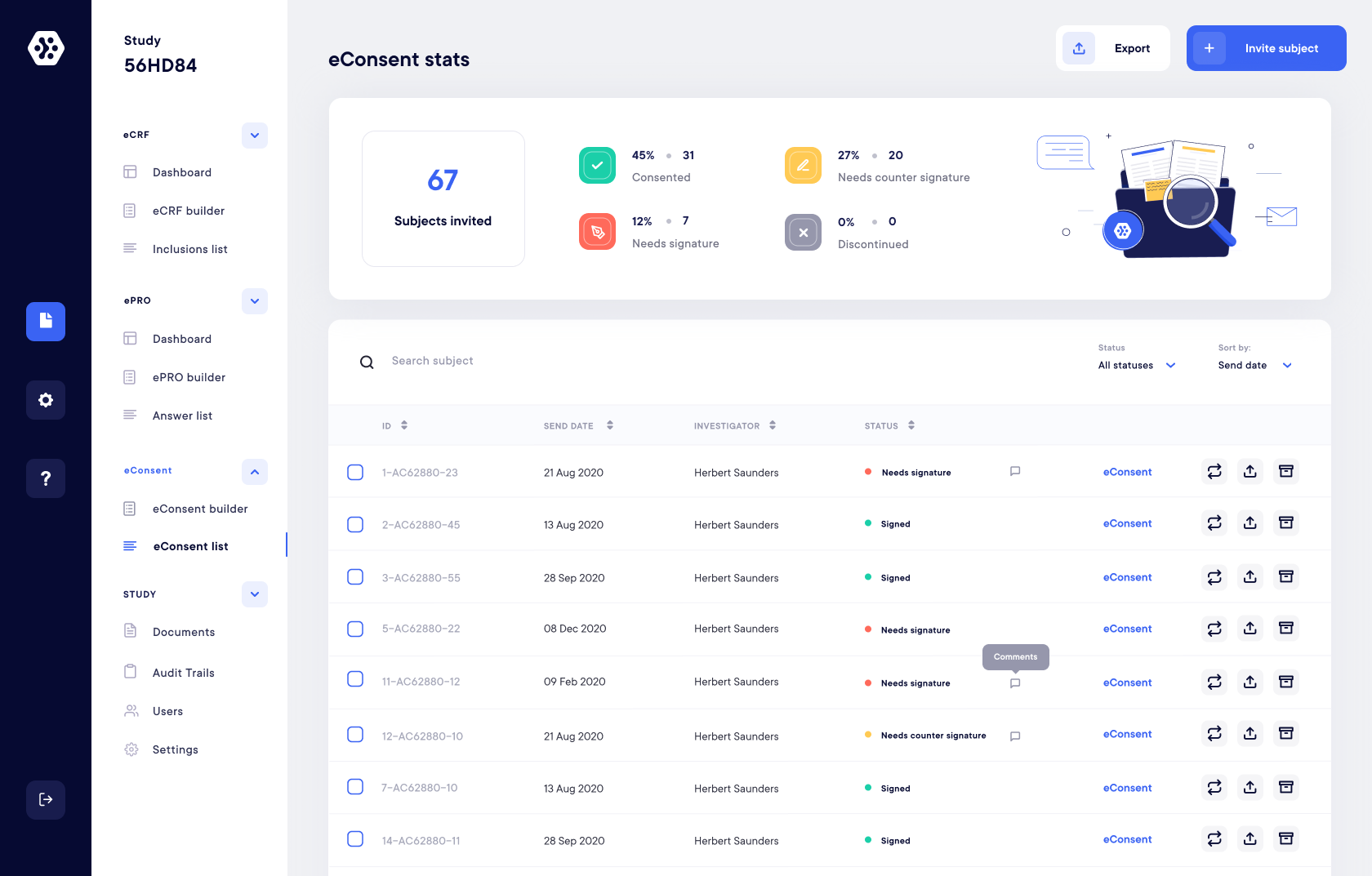
eConsent
Secure Electronic Informed Consent
Take advantage of a secure and reliable eConsent solution for your informed consents. You are just a few clicks away from recruiting and starting your study!






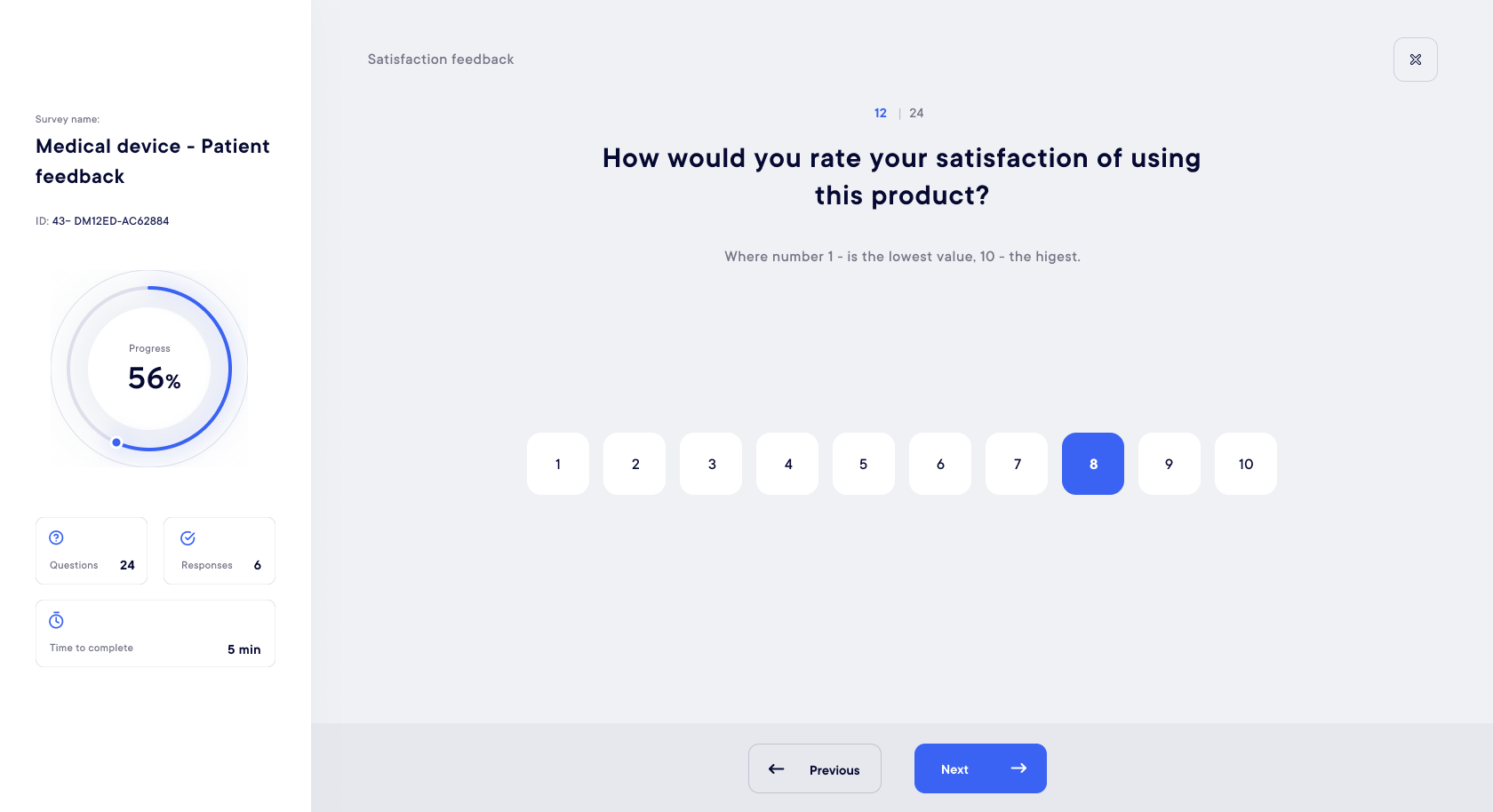
ePRO - eCOA
New generation participant questionnaires and surveys
Discover a tailored, easy-to-use, user-centric survey solution (ePRO) to increase participant engagement and collect real-life data : feedback, evaluation, clinical experience…
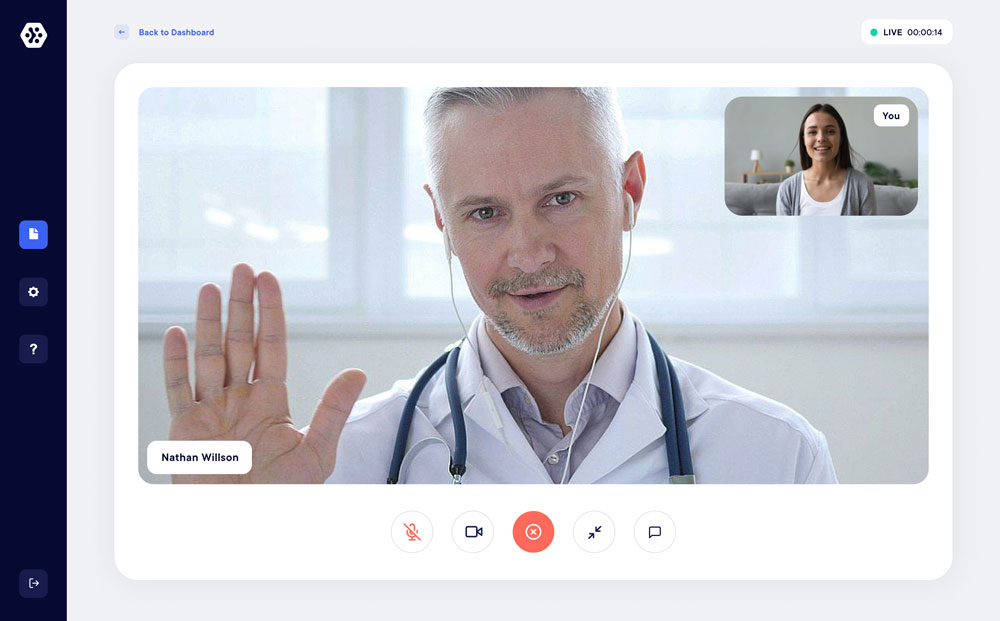
eConsult
Teleconsultation and personalized participant follow-up
Access to high performance HD teleconsultation. Follow up with your participants in complete security and improve their engagement. Identity verification, support for signing electronic informed consent, real-time data collection…
CTMS
The complete CTMS solution for your business.
A unique platform to manage, recruit and engage your patients. Datacapt CTMS solutions help patients stand out of the crowd based on your study criteria.
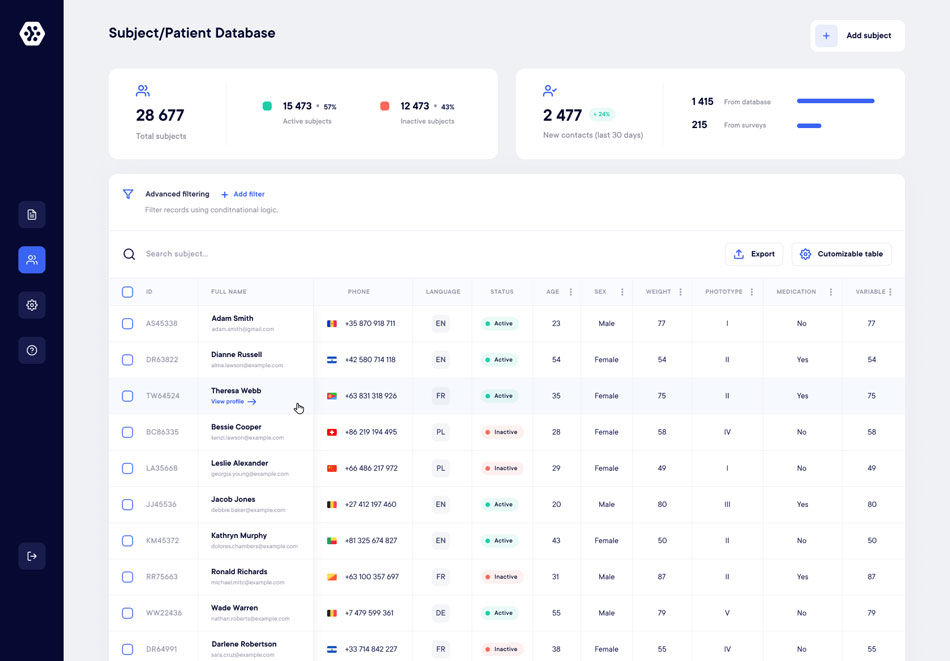
Discover the enhanced efficiency of your trials with Datacapt.
Adopt an effective clinical trial management solution now.
Try for free


