Datacapt eCRF and eSource is an all-in-one eClinical solution designed for modern clinical trials. With a clean and intuitive interface, we provide enhanced accessibility and flexibility empowering researchers to conduct clinical research efficiently. Collect, access, manage, review, and share clinical trial data from any device, at any time!
eCRF - eSource
(Electronic Data Capture)
Datacapt’s eCRF
make it Easier, Faster, and Scalable.
Experience the next level!
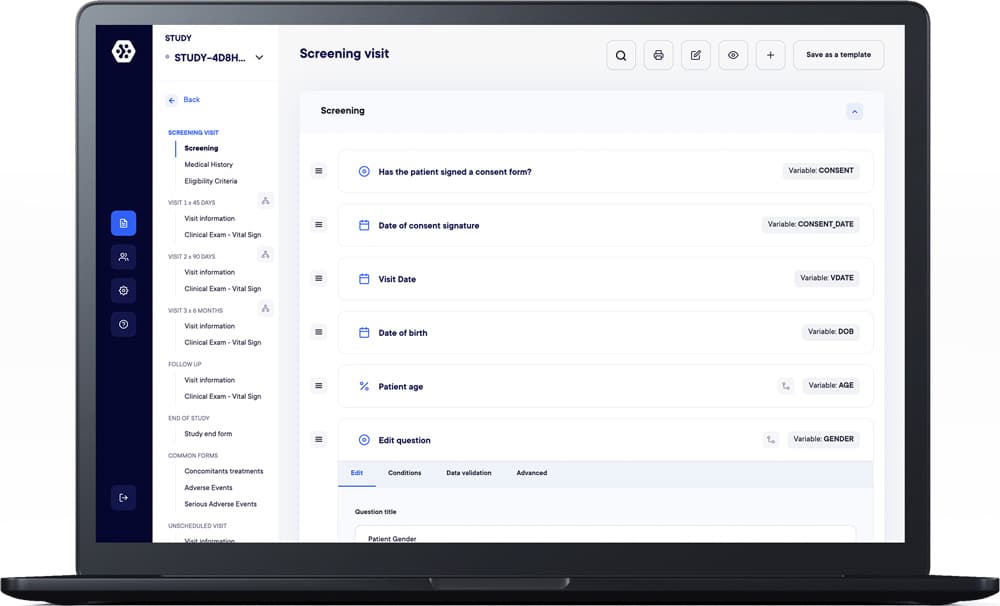
Design eCRFs effortlessly with our 100% no-code builder. Utilize 25+ field types, complex calculations, real-time tests, and high customization, all in a user-friendly interface for quick and powerful eCRF creation.
Save valuable time and deploy faster!
Easily implement edit checks and visibility conditions, from simple to complex, without any coding skills. Validate data effortlessly cross forms/visits, reducing errors. Create professional studies quickly. Take advantage of templates, saving time and effort.

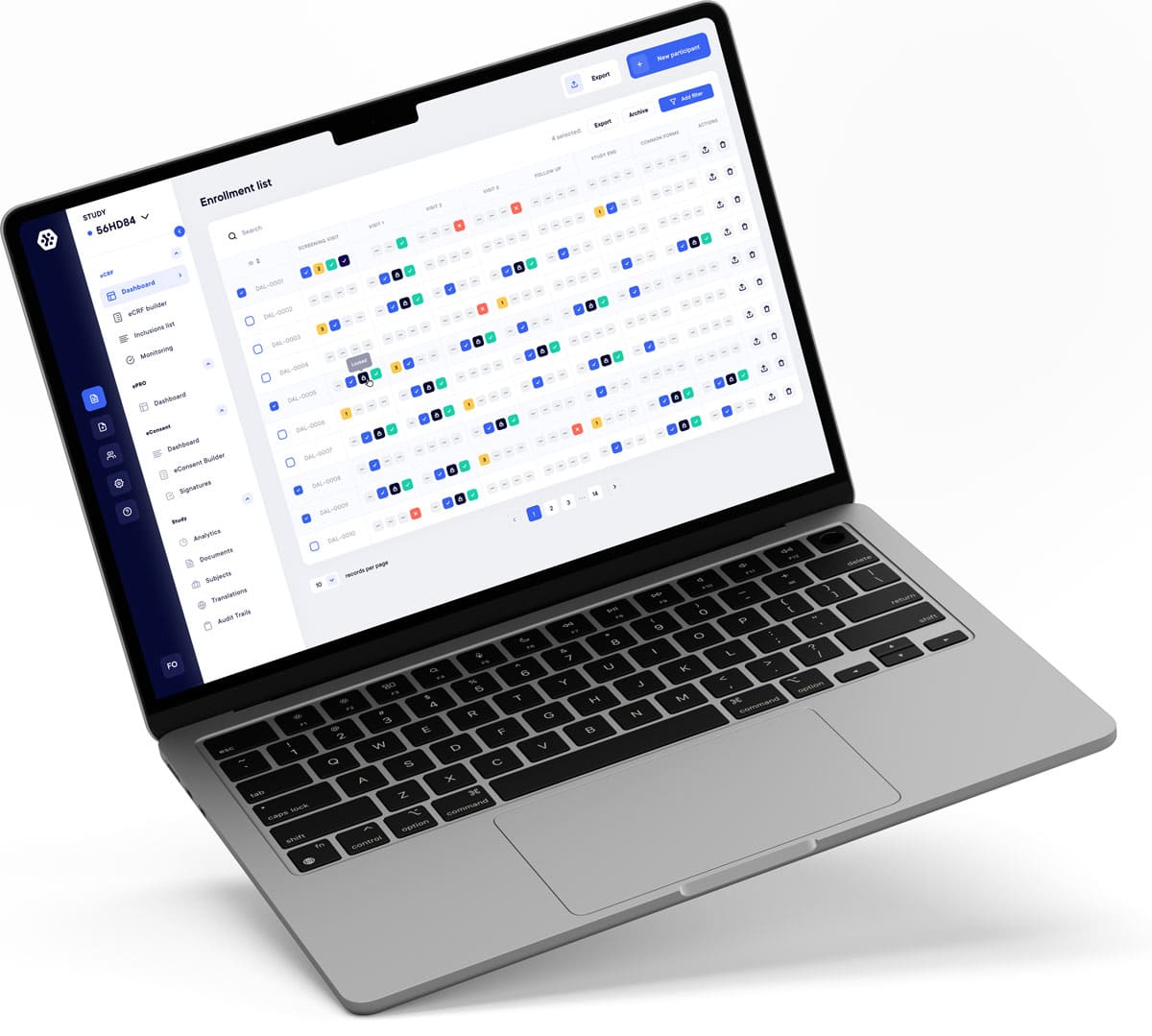
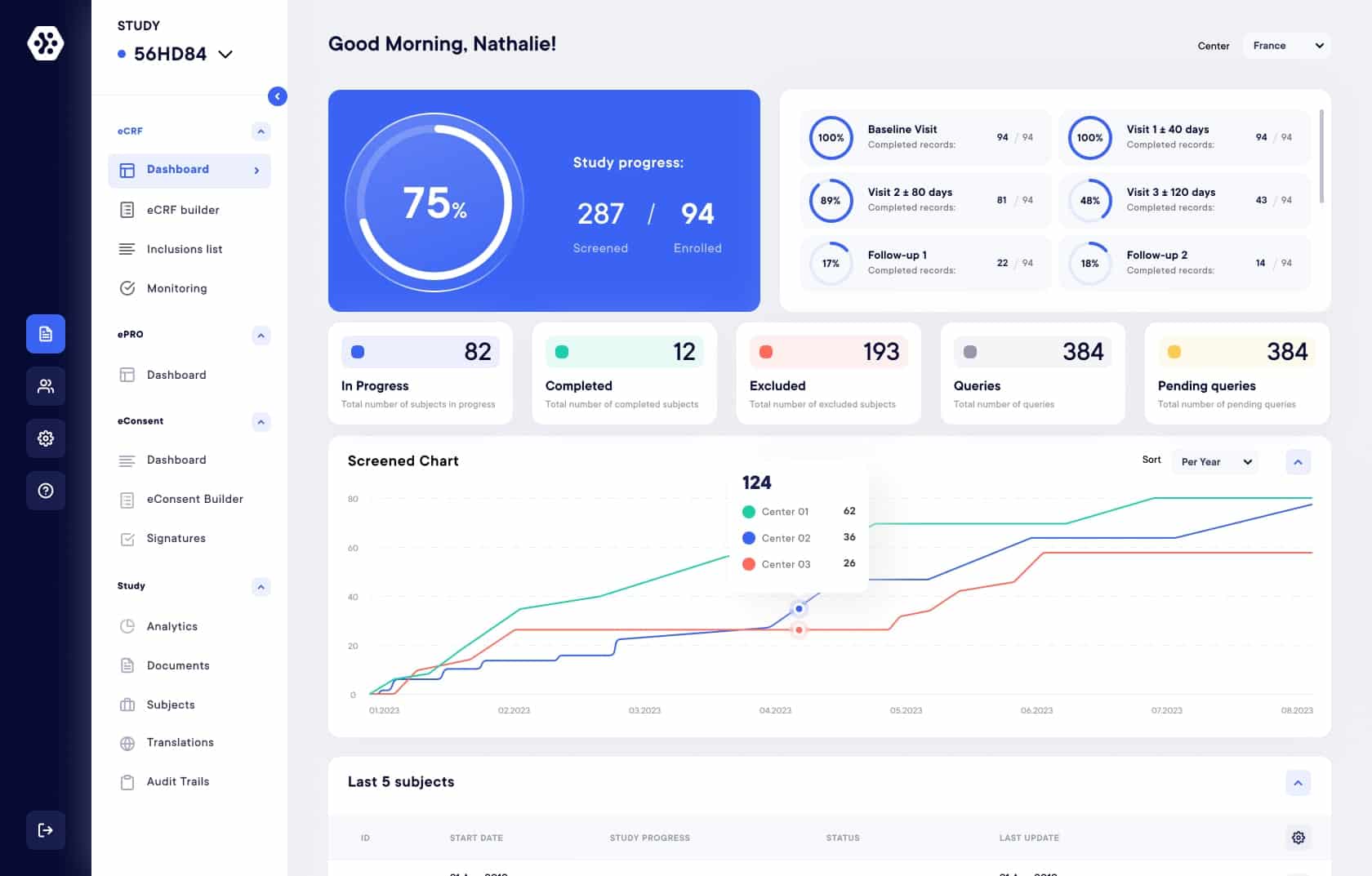
Streamline data collection
Experience the power of our user-friendly interface, revolutionizing your data collection process like never before. Easily manage your subjects and their progress.
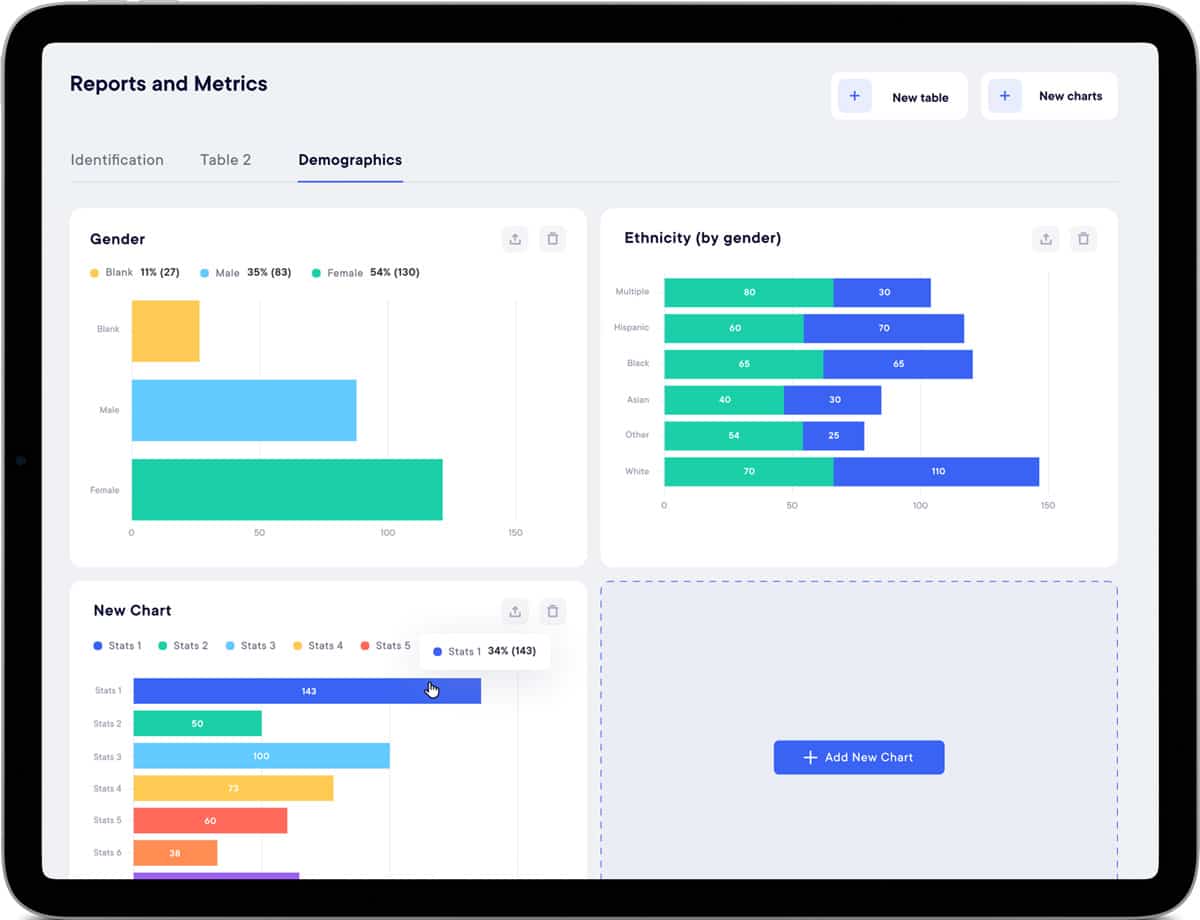
Get real-time reports and metrics
Real-time study progress and metrics, gaining valuable insights. Access standard or custom reports for in-depth data understanding and analysis per subject or center. Make data-driven decisions with confidence. Export data in multiple formats (Excel, CSV, PDF, SAS).

Simplify your monitoring.
Access advanced and comprehensive monitoring features such as data review, queries, signatures, source data verification (SDV), and data locking, among others.
There’s more…
100% autonomous
Invite users, assign roles, and manage permissions seamlessly with our user-friendly interface. Enjoy a smooth and fast experience at every step without any help.
One unified Platform
Experience the most intuitive and comprehensive eClinical platform in the industry. Our flexible, modular platform offers fully integrated solutions such as ePRO, eConsent, Televisits, CTMS, RTSM, and much more…
Compliance and Security
Our platform is ISO 27001, 21 CFR PART 11, and GDPR compliant. Backups are taken every 4 hours. Data is replicated in real-time in a paired region. An external full backup is performed every 24 hours in a third region.
And so much more.
Protocol amendments
Randomization
APIs
Automation and alerts
+85 languages
99,99% uptime and Backups
We can make your clinical trials more efficient!
Start transforming your clinical trials experience with Datacapt Platform.



