Experience faster and improved patient recruitment with eConsent. Upgrade to eConsent, streamlined processes grant better access to studies while enhancing patient engagement, ensuring a seamless and informed consent experience.
Greater participation, and improved data quality.
On-site, hybrid and
remote eConsent.

Utilizing eConsent modernizes and enhances the informed consent process, ultimately benefiting both participants and researchers.
Better
informed
eConsent engages participants with multimedia, simplifying complex information. Participants navigate content at their pace, leading to improved informed decision-making.
Accelerate
enrollment
eConsent streamlines comprehension, signing, and tracking, reducing manual handling. It simplifies both the review and enrollment processes.
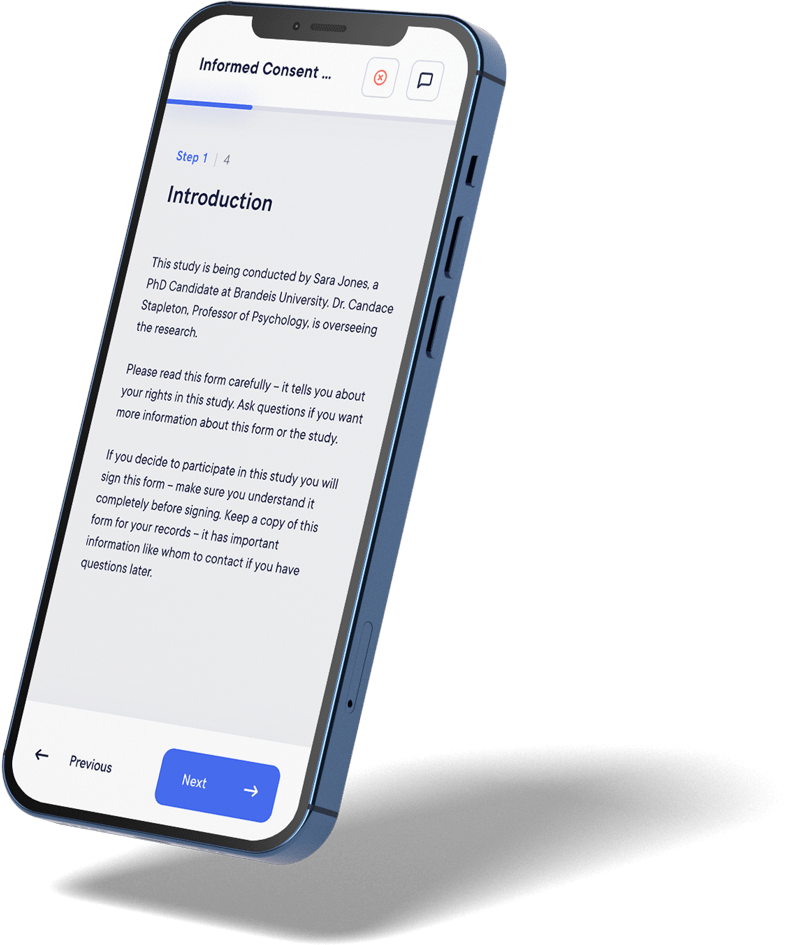
User-Friendly
interfaces
Datacapt eConsent provides intuitive interfaces, interactive elements, and guided navigation for easy participant comprehension and user-friendly step-by-step processes.

Gain real-time visibility
across all your sites
Real-time tracking and monitoring empower coordinators to guide patients through enrollment stages, ensuring compliance and timely support. Enhanced engagement aids adherence to regulations, fostering well-managed clinical trials.
A simple
user experience
Accessible from any device (BYOD) Datacapt eConsent ensures a seamless experience anywhere in the world. Participants effortlessly review, sign, and engage with consent materials, enhancing accessibility and convenience in the process.
Televisits for remote eConsent
Televisits enable remote eConsent, allowing participants to review and sign consent forms from any location, enhancing convenience, accessibility, and study enrollment efficiency.
User management
Introducing adaptable roles to streamline consent workflows and manage multicenter studies efficiently. Choose custom or predefined roles: Study Admin, Data Manager, Site Admin, Investigator, Monitor, Read-only users.
Compliance
Designed to comply with global regulations, including FDA 21 CFR Part 11, ICH GCP, and GDPR, eConsent digitizes and secures consent processes for accuracy, accessibility, and auditability.
Need more info?
Check out our most frequently asked questions.
Start Your Journey with Datacapt eConsent
Boost Enrollment Efficiency with Datacapt eConsent.



