of clinical trials are shut down due to a lack of patients. That insanely high number might surprise you or make you despair. It is a sad reality, knowing that the vast majority of study budgets go to patient recruitment.
But what is the cause of it?
And how to reverse this stat?
We have listed multiple reasons why your clinical studies are lacking patients and the solutions to overcome them.

Lack of awareness
One of the principal reasons why patients aren’t engaging in clinical trials is because of a lack of awareness. Indeed most patients aren’t aware of what is a clinical trial. Patients underestimate the utmost importance of taking part in clinical trials. There is a huge lack of information on this subject which have to be filled by spreading awareness of clinical trials.
It can occur thanks to better marketing strategies promoting trials, and better communication from clinicians and doctors.
Fear of the unknown/Adverse Events
Participating in a clinical trial may sound scary. In fact “clinical trial” hasn’t a positive connotation. Which can lead to people being afraid to enroll.
By saying clinical trials, most people will think about them being rat labs. That the treatment they will be trying is unsafe and shady. Also, patients apprehend the possible adverse events they might face. Indeed when getting enrolled in a clinical trial there might be some possible side effects, that are scaring the patients away.
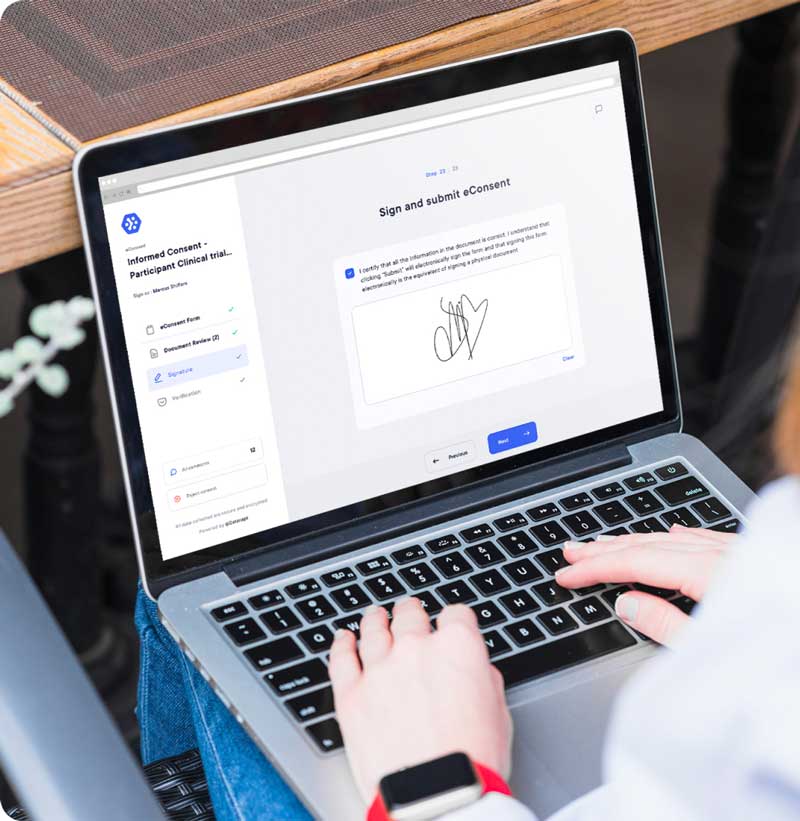
One solution is to inform your patients as much as possible about what the trial consists of. What will be tested, if adverse events are possible and how you will handle them. Using digital consent also allows for better understanding thanks to charts, photos, and videos… which can bring more clarity.
Finally, having direct communication with your patients, and taking the time to address their concerns is key in the enrollment process.
Inconvenience
Engaging in a clinical trial is difficult enough, but using paper forms adds up even more difficult and discourages your patients.
Having to fill multiple sheets for consent, complete surveys by hand… It is out of fashion!
Your patients might lose patience and also forget to enter some data.
In order to avoid that, the use of digital forms is of great help to both the center and volunteers. Digital forms such as ePRO or eConsent allow the patients to complete questions on a smartphone, computer, or tablet. Accessing it 24h/7 from everywhere in the world. It gives them the flexibility to answer their surveys from the comfort of their home and manage their time at their own convenience.
Learn more about the eConsent
Restrictive inclusion criteria
Another reason why clinical trials are lacking patient admission is too restrictive inclusion criteria. Indeed, when designing a clinical trial, clinicians might sometimes focus on a too-small target population. Because of it, many people won’t be able to join the study. Also, sometimes when a patient qualifies for enrollment, his condition is stopping him from it.
Chances of receiving a placebo
When signing up for a clinical trial in the pharmaceutical area, people are hoping to try a new treatment in order to benefit from it. However, because clinical trials are randomized some patients will receive a placebo instead of the real treatment.
This is an obstacle for patients has they are enrolling hoping to receive the treatment.
However, except in Phase 1 trials, treatments are compared to already existing medications, so the patients will still receive treatment.
Patients effort
When engaging in a study, patients must put in effort in order to keep being part of the trial. It takes the form of multiple backs and forths between his private life and the study center to visit the doctor. Patients often have to drive several hours to reach the center. Also, once at the center, they are required to wait for the clinician. Most people have full-time jobs which makes it difficult to get daily appointments in the middle of the day.
Also, some patients might find it stressful to come due to difficulty in moving around (elderly or patients with small children).
In order to remove the thorn in the side, is to implement remote forms in your study. Indeed, using ePRO avoids your patients to come on-site on a regular basis. It gives them the possibility to enter data daily and for you to collect data in real-life.
Learn more about the ePRO
Lack of information
Patients aren’t enough informed about the clinical trial possibilities. Indeed, they rely on doctors or clinicians to indicate to them that a clinical trial is available. People aren’t inclined to check by themselves.
Healthy people aren’t much informed either that trials are taking place in the cosmetic field and that they can enroll whenever they want.
To get rid of this lack of information, center, doctors, and laboratories must spread the word. Also, in order for people to engage in clinical trials, the patient experience must be optimal. This is done by putting implementing a new way of collecting data: digitalizing the processes to make them more flexible, convenient, and secure.
Datacapt possess all the advantages cited above for your patients.
Datacapt has created a special interface for patients to connect to. Thanks to this 100% secured interface (password and id), patients have access to all their documents and surveys. They can access it at any time and from any place.
This personal interface is viewable on smartphones, tablets, and computers. It allows for better flexibility and better patient retention.