Faster, more practical and just as secure as a paper document, electronic-consent have been developed since the early 2000s, in particular thanks to the law of June 21st 2004 on confidence in the digital economy.
eConsent now makes it possible to ensure that potential patients or volunteers are fully informed, despite the multitude of information provided, traditionally on paper.
Main benefits
A law couldn’t be enough to promote this new way of working. Electronic consent has many benefits and is also more environmentally-friendly as it doesn’t require printing or transport:
- It prevents a document from being lost on its way to the recipient.
- It allows you to have only original documents.
- It’s quick to create and set up.
- It avoids errors and allows for faster and remote verification.
- It provides better information for participants to enable them to make informed decisions through interactive multimedia elements.
- It ensures greater exchanges reliability, traceability and security.
- It is perfectly suited to decentralized and multi-centers studies.
- It reduces costs because once the contract is drawn up, it only takes two or three minutes to complete the signatures, ensures its sending and reception by the various recipients.
It is increasingly coupled with the use of teleconsultation and images, sound, video and tick-boxes to facilitate the consent process and remote participant’s understanding. The eConsent provides easy access to legal information and various patient concerns, improving patient empowerment, retention and compliance, especially in decentralized studies
It has been boosted by the COVID 19 pandemic. Used worldwide, the eConsent is becoming the standard for clinical research
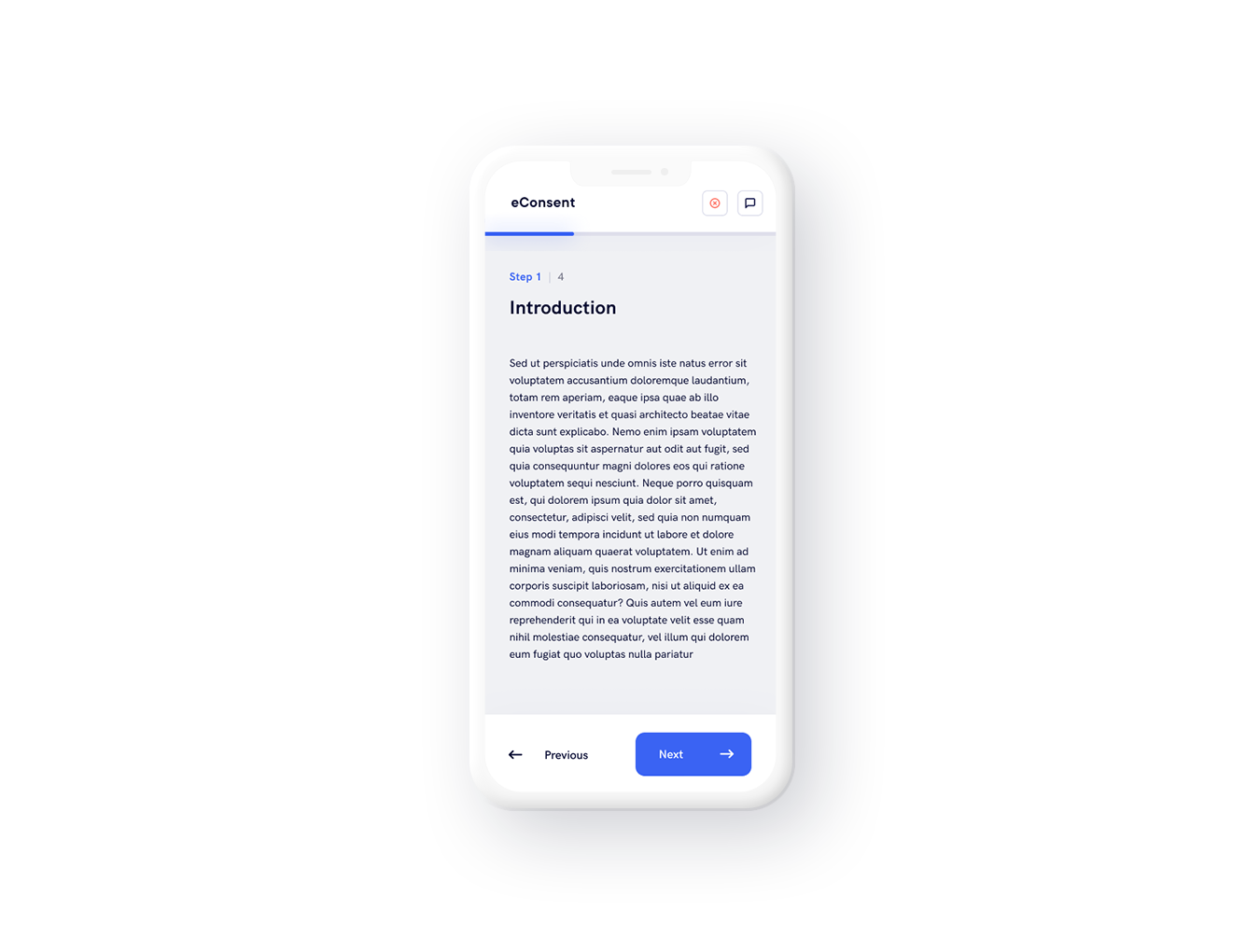
Implementation methods' and use
A document that has to be signed electronically must, as stated in the GDPR, be explicit, free and informed.
It may only be used by person of full mental capacity and may contains legal paragraphs that don’t contravene public policy.
A user profil is created with strict access rules to ensure confidentiality and process security.
Document validation requires the creation of electronic signatures which identify each signatory and guarantee, when used, the persons’ consent to the obligations listed in the act just signed.
The consent must also be dated and then stored and archived in conditions that guarantee its integrity. Each signatory must be sent a copy in electronic form.
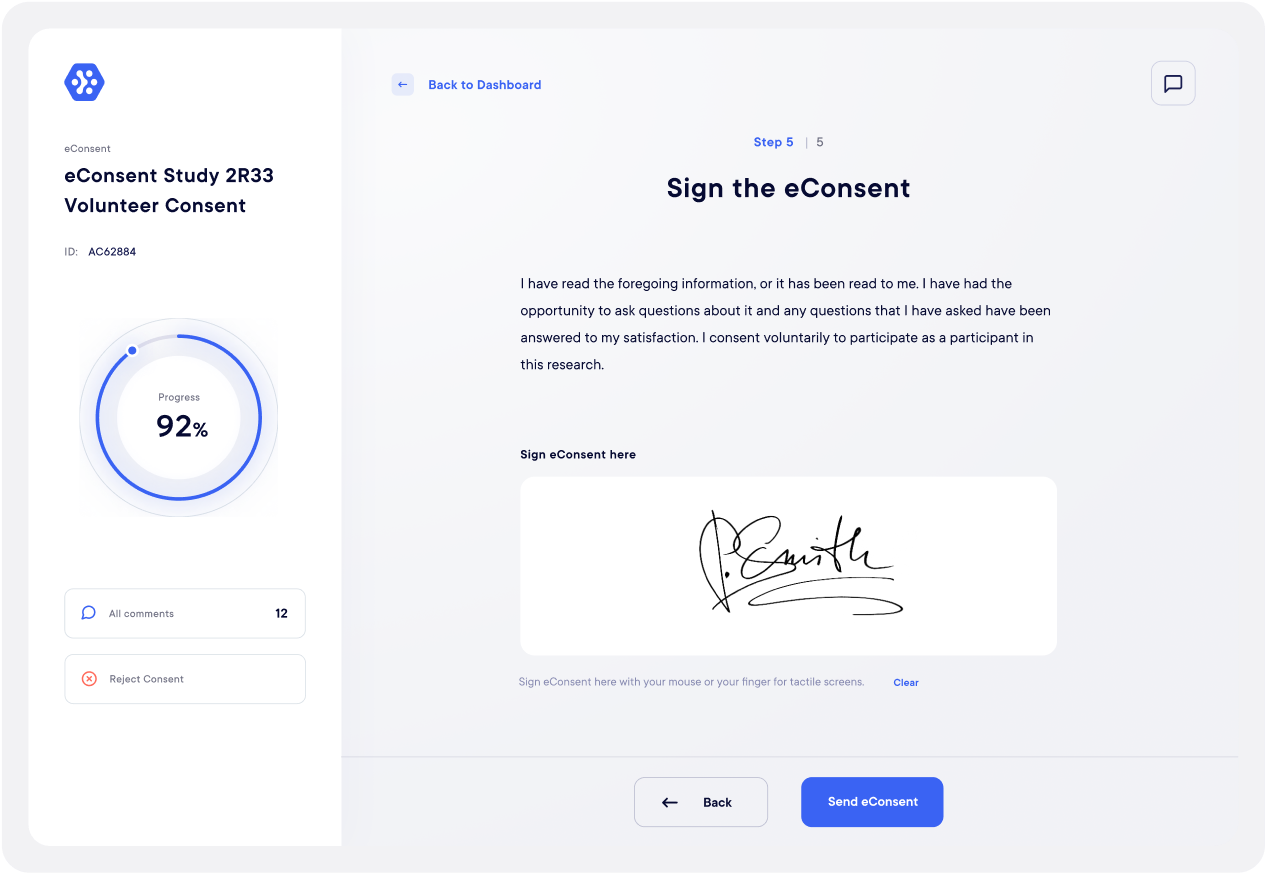
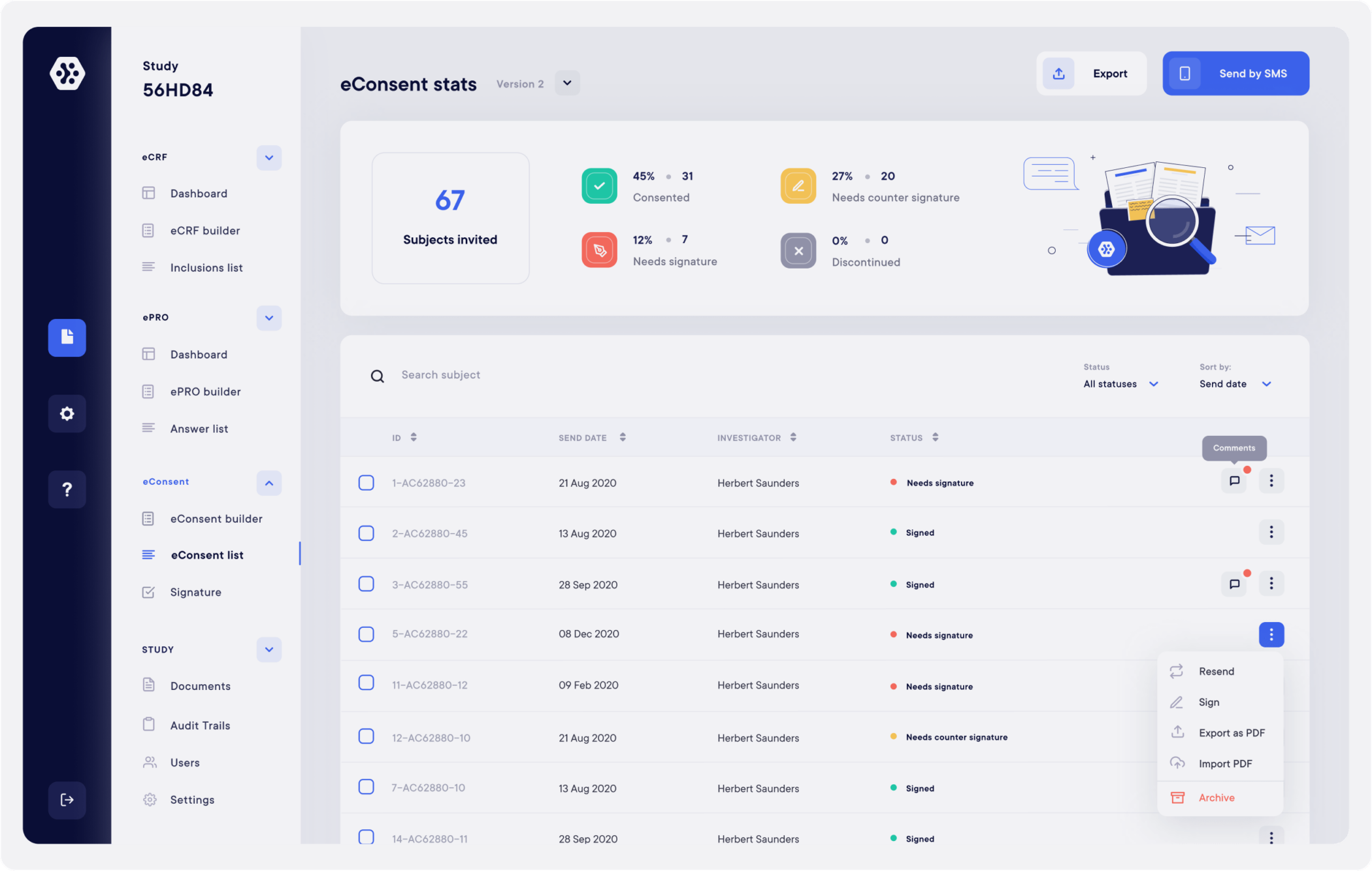
Datacapt's eConsent solution
Datacapt’s eConsent naturally meets all the legal requirements and offers all the necessary advantages for a smooth signature process. It is particularly flexible and intuitive for the various users and allows real-time signatures’ monitoring.
Building and sending electronic consent is fast, smooth and secure. Each participants has access to an interactive, engaging and informed consent experience.
It facilitates the investigator’s site work, who can have more time for other tasks such as data control and patient safety.
It ensure the perfect documents’ security, essential to clinical studies and allows a much better costs’ control.
Would you like to receive more information?
Discover Datacapt’s eConsent free of charge
and manage your studies with complete peace of mind.




