Leading and emerging players in clinical trials
place their trust in us.
Discover how companies utilizing Datacapt are accelerating the launch of tomorrow’s innovative treatments and products.
Become a part of this change. Join them now!
Next-generation builder
Create your eCRF forms with a Drag & Drop in minutes. Create and re-use your forms.
Use more than 25 field types in your forms to make them fit your exact needs.
Add logic and data validation without any coding. No more need for technical skills, start building immediately.
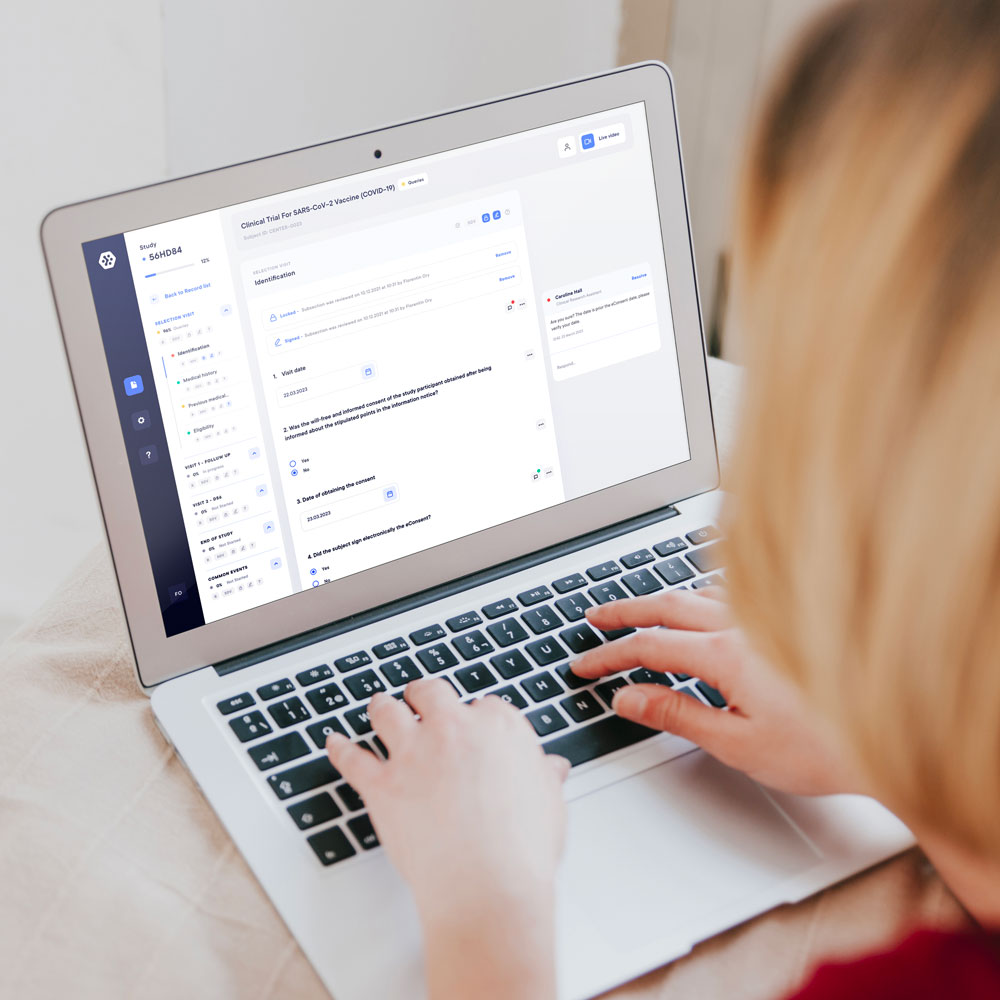
Follow the progress and monitor in real-time
Now it’s easier than ever to review, control, SDV, lock, and sign your eCRF!
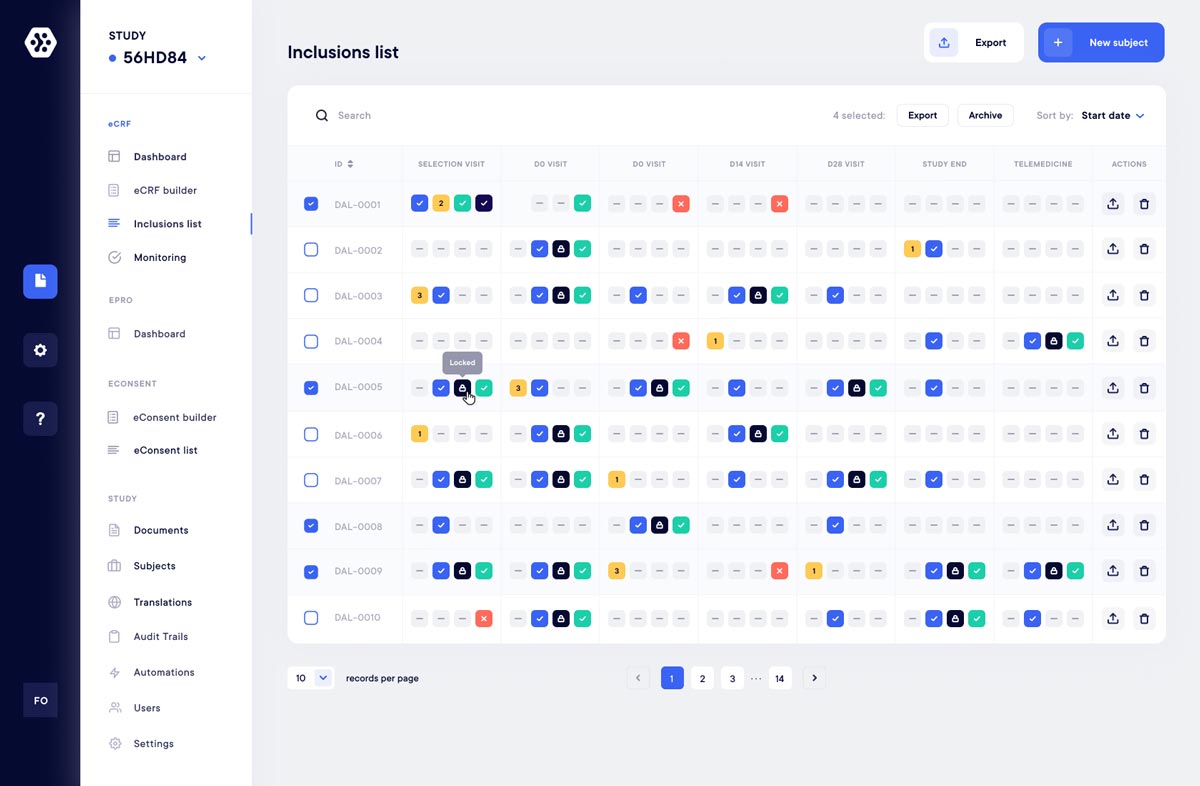
Visualize and export your data easily
Export your data in various formats such as Excel, CSV, or PDF, CDISC Compliant.
Be even more secure
A secure platform that improves data security and quality.

Frequently asked questions
Where is stored the data?
The security is our priority. We have 3 different zones in the world: USA (N.Virgnia and N.California), Europe (Paris and Ireland) and China. Datacapt is fully compliant with 21 CFR PART 11, HIPAA, GDPR, Good Clinical Practice (ICH-GCP) and certified ISO 27001 and HDS (Health Data Hosting). We have external backups every 24 hours and backups every 6 hours.
In case of a problem can I contact technical support?
Our technical support is available at any time to answer your questions 24/7. You can reach us at the following address: [email protected]. We respond with an average time of 1 hours.
Does Datacapt require coding skills?
You don’t need any coding skills to use Datacapt as it has been built to be user-friendly. The platform is very easy to use and you’re 100% autonomous from the creation of your studies to the database lock and the data export.